Abstract
Background: For most acute myeloid leukemia (AML) patients an allogeneic hematopoietic stem cell transplantation (HSCT) offers the highest chance of cure and long-term survival. Higher age and/or comorbidities may confine this option in a considerable number of AML patients due to toxic myeloablative preparation regimens. Non-myeloablative conditioning (NMA) regimes were developed to allow HSCT in these patients. The HSCT comorbidity index (HCT-CI) has been shown to predict outcome after allogeneic HSCT in cohorts of unselected patients. However, there is no separate evaluation of its prognostic significance in older (> 60 years) AML patients undergoing less-toxic NMA-HSCT. We evaluated the prognostic impact of the HCT-CI in a large, homogenously treated AML patient cohort that received NMA-HSCT.
Methods: We retrospectively analyzed 289 AML patients older than 60 years (median age 66, range 60-77 years) at the time of NMA-HSCT. The conditioning regimen consisted of fludarabine 30 mg/m 2 for 3 consecutive days in addition to 2 (n=278) or 3 Gy (n=6) total body irradiation (TBI), five patients received 2 Gy TBI alone. Graft versus host disease prophylaxis contained cyclosporine and mycophenolate mofetil, none of the patients received in vivo or in vitro T-cell depletion. Disease risk at diagnosis was assessed according to the European LeukemiaNet (ELN) 2017 classification. In patients transplanted in morphologic remission, measurable residual disease (MRD) at HSCT was assessed based on NPM1 mutations and BAALC, MN1, and WT1 expression levels. The pre-transplant HCT-CI was calculated prior to the start of the conditioning regimen. Median follow up after HSCT was 3.8 years.
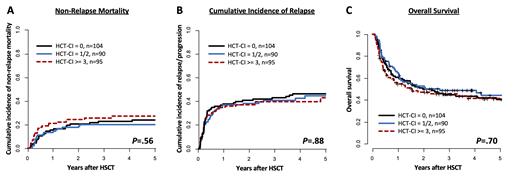
Results: 97% of the patients (n=281) engrafted. 36% of the patients (n=104) had a low risk (0 points), 31% (n=90) an intermediate risk (1-2 points), and 33% (n=95) a high risk (≥3 points) HCT-CI score. The performance score at HSCT (ECOG, 0/1 vs. 2/3) was not different between HCT-CI risk groups (P=.86). Incidences of chronic graft-versus-host disease (GvHD) were 16% limited and 52% extensive disease. The non-relapse mortality (NRM) did not differ significantly between HCT-CI risk groups after NMA-HSCT (P=.56, Figure 1A, at 5 years HCT-CI low 24% vs. HCT-CI intermediate 20% vs. HCT-CI high 27%, respectively). Likewise, neither the cumulative incidence of relapse (P=.88, Figure 1B, at 5 years 46% vs. 45% vs. 43%) nor the overall survival (P=.70, Figure 1C, at 5 years 40% vs. 44% vs. 41%) differed according to the HCT-CI risk groups. The HCT-CI also did not impact outcomes in separate analyses according to ELN2017 risk at diagnosis (OS, P=.20, P=.30, and P=.70 for favorable, intermediate, and adverse, respectively) or MRD status prior to HSCT (OS, P=1 and P=.30 for MRD-negative and MRD-positive patients, respectively). In the favorable risk subgroup of patients being MRD-negative at the time of NMA-HSCT, the median overall survival reached 49% at 5 years after NMA-HSCT, irrespective of the HCT-CI.
Conclusion: In the here presented cohort of older AML patients a higher HCT-CI did not have a negative impact on NRM or survival. In general, the NRM following NMA-HSCT was relatively low. Our data indicates that comorbidities per se - reflected by a higher HCT-CI score - should not impede NMA-HSCT. Independently from the HCT-CI score MRD-negative patients had notably good survival of 49 % at 5 years following NMA-HSCT. As the incidences of chronic graft-versus host disease were relatively high, alternative immunosuppressive strategies may help to further improve outcomes. Our data aid in informed clinical decisions regarding HSCT consolidation in older AML patients since we show that HSCT with this reduced toxicity regimen represents a feasible treatment option in older and comorbid AML patients also with higher HCT-CI scores.
Backhaus: Bayer: Other: Current Employment of Family Member. Jentzsch: Astellas: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria. Vucinic: Janssen: Honoraria, Other: Travel Sponsoring; Novartis: Honoraria; Gilead: Honoraria, Other: Travel Sponsoring; Abbvie: Honoraria, Other: Travel Sponsoring; MSD: Honoraria. Franke: BMS: Honoraria; Gilead: Honoraria, Other: Travel Sponsoring; Jazz Pharmaceuticals: Honoraria, Other: Travel Sponsoring; MSD: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Platzbecker: Celgene/BMS: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Geron: Honoraria; Takeda: Honoraria. Schwind: Novartis: Honoraria, Research Funding; Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal